Hims & Hers abandons proposal to create imitation of Novo Nordisk's latest Wegovy weight loss medication
Hims & Hers Withdraws Plan for Generic Wegovy Pill
Telehealth provider Hims & Hers has decided to halt its initiative to introduce a generic version of the weight-loss medication Wegovy. This reversal came just two days after the company announced the new product, following warnings from the Food and Drug Administration (FDA) about restricting access to the ingredients used to replicate widely used weight-loss drugs.
On Thursday, Hims revealed plans to offer a compounded version of the newly released Wegovy pill, which pharmaceutical company Novo Nordisk launched last month. In response, Novo Nordisk threatened legal action, and the FDA announced on Friday its intention to take firm measures to limit access to the active ingredients in popular GLP-1 medications such as Wegovy, Ozempic, and Zepbound.
Despite the announcement, Hims' website continued to promote the new semaglutide pill on Saturday afternoon, even after the company stated on X that it would discontinue the product. Semaglutide is the active ingredient in Wegovy.
"Since launching the compounded semaglutide pill on our platform, we’ve engaged in productive discussions with various industry stakeholders. Based on these conversations, we have chosen to stop offering this treatment," Hims said in a statement. "Our commitment to providing safe, affordable, and personalized care to millions of Americans remains unchanged."
As of Saturday, Hims had not clarified whether it would alter its compounded injectable weight-loss medications in light of the FDA's recent actions.
Background and Pricing Strategy
The San Francisco-based company had intended to offer its version of the Wegovy pill at a much lower price than Novo Nordisk, charging $49 for the first month and $99 for subsequent months, compared to Novo's $149 monthly price. Hims and similar companies initially gained traction by selling affordable generic treatments for conditions like hair loss and erectile dysfunction, before expanding into the lucrative obesity drug market.
Industry Developments
Novo Nordisk is set to promote its FDA-approved Wegovy pill with a star-studded Super Bowl commercial on Sunday. The company did not immediately respond to Hims’ decision to withdraw its generic offering. Meanwhile, competitor Eli Lilly anticipates FDA approval for its oral orforglipron weight-loss drug later this year, but Wegovy remains the first pill of its kind available to consumers.
Regulatory and Market Context
The compounded medication Hims planned to sell had not received regulatory approval and lacked clinical trials to prove its effectiveness.
The FDA allows specialty pharmacies and companies to create compounded versions of branded drugs during shortages. The surge in demand for GLP-1 medications in recent years has led companies like Hims to enter the multibillion-dollar market, with many patients opting to pay out of pocket.
In 2024, the FDA declared that GLP-1 drugs were no longer in short supply, which was expected to curtail compounding practices. However, companies like Hims have continued to offer their versions by utilizing exceptions that permit compounding when prescriptions are tailored to individual patients.
Disclaimer: The content of this article solely reflects the author's opinion and does not represent the platform in any capacity. This article is not intended to serve as a reference for making investment decisions.
You may also like
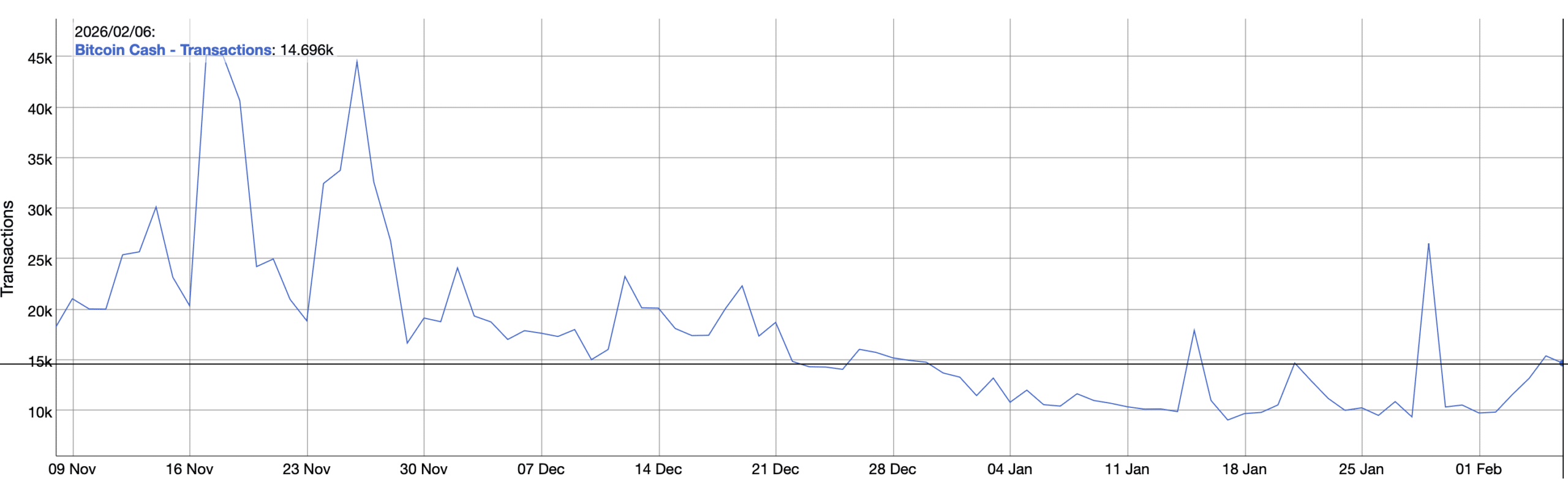
Bitcoin Cash’s rally faces KEY test – Can BCH hold above $500?
Facing Legal Threats, Hims & Hers Removes Imitation Weight Loss Drugs
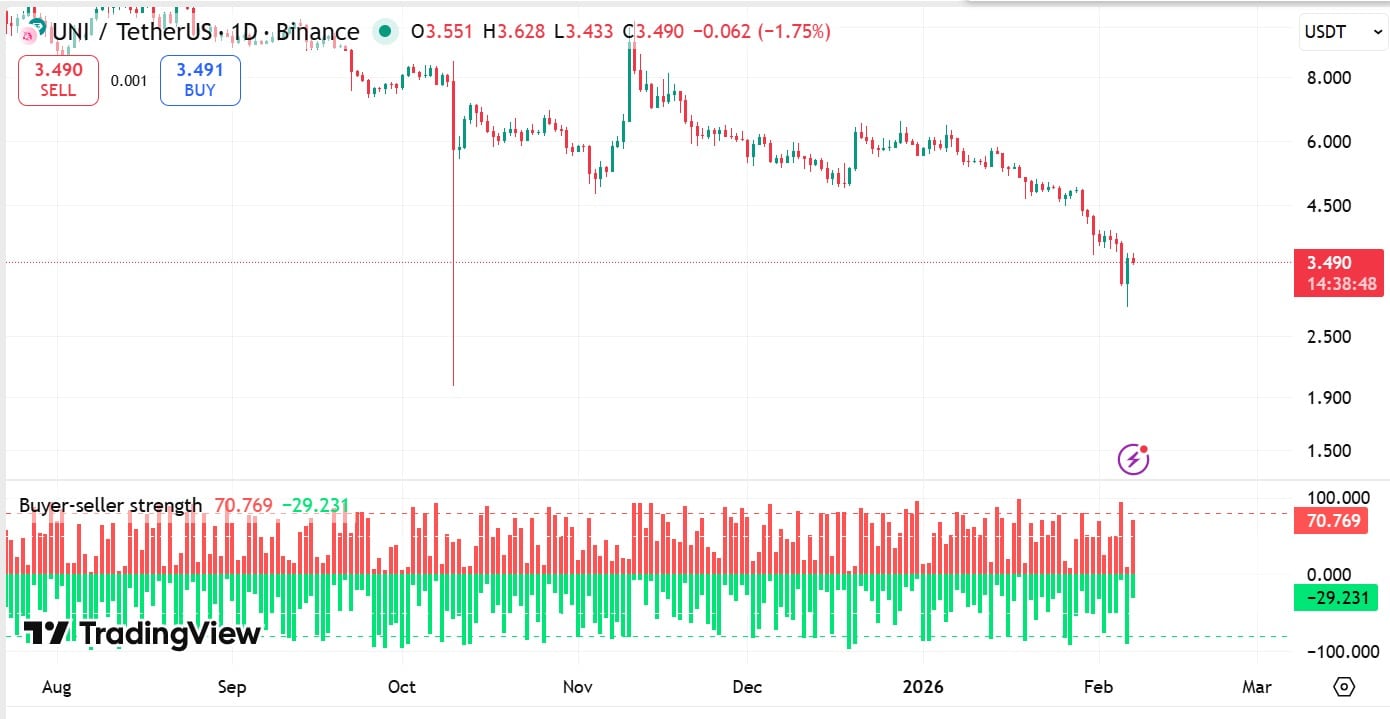
Uniswap rebounds: Can UNI push past $4.2 EMA resistance?
Apollo, BlackRock, and Oaktree Request Judge to Dismiss Altice Lawsuit
