Krystal Biotech Q4 Earnings Beat Estimates, Revenues Miss Mark
Krystal Biotech KRYS reported fourth-quarter 2025 earnings per share (EPS) of $1.70, which surpassed the Zacks Consensus Estimate of $1.62. The reported EPS was up from $1.52 in the year-ago quarter.
Revenues of $107.1 million increased 17.5% year over year in the reported quarter but missed the Zacks Consensus Estimate of $109 million. Revenues came in solely from the sales of Vyjuvek.
The FDA approved Krystal’s lead drug, Vyjuvek, the first-ever revocable gene therapy, in 2023 for treating patients aged six months or older with dystrophic epidermolysis bullosa (DEB), a rare and severe monogenic disease that affects the skin and mucosal tissues. The drug has also received approval from the FDA for the treatment of DEB patients from birth, with authorization for at-home administration by patients or their caregivers.
The company secured more than 660 reimbursement approvals for Vyjuvek in the United States, supporting nationwide access. Internationally, robust patient demand continues to drive steady uptake following the launches in Germany, France and Japan, with more than 90 patients being prescribed the therapy across these markets.
Over the past year, shares of KRYS have risen 63.4% compared with the industry’s 19.3% growth.
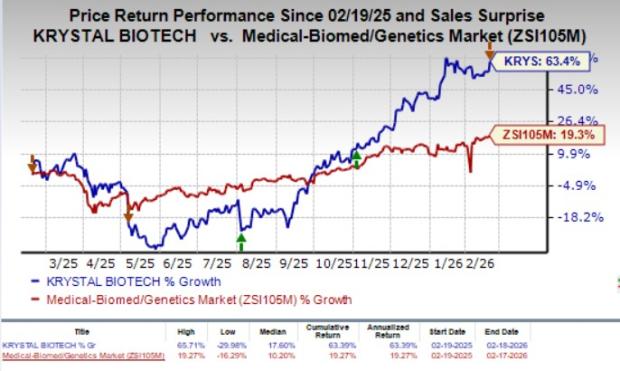
Image Source: Zacks Investment Research
KRYS’ Q4 Earnings in Detail
The top line comprises product revenues from Krystal’s only marketed drug, Vyjuvek.
Krystal generated $107.1 million in product revenues from Vyjuvek, up from $91.1 million in the year-ago quarter, driven by strong patient uptake.
The gross margin in the reported quarter was 94%.
Research and development (R&D) expenses were approximately $14.8 million, including stock-based compensation, up 9.36% year over year. Selling, general and administrative (SG&A) expenses totaled approximately $41.4 million, including stock-based compensation, up 32.5% from the year-ago level. This increase was primarily due to increased headcount, legal and consulting services and marketing costs to support the global launches of Vyjuvek.
As of Dec. 31, 2025, cash, cash equivalents and investments totaled $955.9 million compared with $864.2 million as of Sept. 30, 2025.
KRYS’ Full-Year 2025 Results
For 2025, KRYS reported total product revenues of $389.1 million, which rose 34% year over year.
For full-year 2025, the company recorded net earnings of $6.84 per share compared with $3.00 reported in 2024.
2026 Guidance
Krystal Biotech expects non-GAAP combined R&D and SG&A expense to range from $175 million to $195 million in 2026.
KRYS' Recent Pipeline Updates
For Vyjuvek, pricing negotiations with reimbursement authorities remain ongoing in Germany and France and are expected to continue through at least the second half of 2026 and 2027, respectively.
Krystal Biotech is also advancing a robust clinical pipeline of investigational genetic medicines in the fields of respiratory, oncology, dermatology, ophthalmology and aesthetics.
On the respiratory front, the company has two candidates in its pipeline — KB407 and KB408.
The company is evaluating KB407 for the treatment of cystic fibrosis (CF). Data from Cohort 3 of CORAL-1, a multicenter dose-escalation study evaluating KB407 in CF patients regardless of genotype, were announced in January. The data confirmed successful lung delivery and expression of functional cystic fibrosis transmembrane conductance regulator protein with inhaled KB407 in cystic fibrosis patients.
The company plans to initiate enrollment in the registrational repeat-dose CORAL-3 study evaluating KB407 for the treatment of CF in the first half of 2026.
KB408 is being evaluated for the treatment of alpha-1 antitrypsin deficiency (AATD) lung disease. Enrollment is ongoing in repeat-dose Cohort 2B of the SERPENTINE-1 study, with interim results expected in 2026.
In the ophthalmology space, another candidate, KB803, is being evaluated in IOLITE, a phase III randomized, placebo-controlled crossover study for the treatment and prevention of corneal abrasions in DEB patients. Krystal Biotech expects to complete enrollment in the first half of 2026, with top-line data anticipated in 2026.
Krystal is also evaluating KB801 for the treatment of patients with neurotrophic keratitis (NK). A registrational, randomized, double-masked, placebo-controlled study, EMERALD-1, is evaluating the safety and tolerability of topical ocular administration of KB801 in patients with NK. Patient enrollment is currently ongoing and top-line data from the study is expected in 2026.
In January, the FDA granted Fast Track Designation to KB111 for the treatment of Hailey-Hailey disease (HHD).
On the oncology front, Krystal has a promising candidate, KB707, which is being developed for the treatment of solid lung tumors.
The FDA granted Regenerative Medicine Advanced Therapy (RMAT) designation to KB707 in February for the treatment of advanced or metastatic non-small cell lung cancer (NSCLC). Krystal is currently enrolling patients in the dose-expansion cohort of its phase I/II KYANITE-1 study, which is evaluating inhaled KB707 as monotherapy and in combination with chemotherapy in patients with advanced lung tumors.
In the aesthetics space, the company’s wholly owned subsidiary, Jeune Aesthetics, is currently developing KB304 for the treatment of wrinkles of the décolleté. The company has aligned with the FDA on a validated décolleté-specific photonumeric scale and expects to initiate a mid-stage study in 2027.
KRYS’ Zacks Rank & Stocks to Consider
Krystal Biotech currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Harmony Biosciences HRMY, Alkermes ALKS and Castle Biosciences CSTL, each currently sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 60 days, estimates for Harmony Biosciences’ 2026 earnings per share have risen from $3.72 to $4.00. HRMY shares have lost 5.5% over the past year.
Harmony Biosciences’ earnings beat estimates in two of the trailing four quarters but missed on the remaining occasion, with the average surprise being 7.20%.
Over the past 60 days, estimates for Alkermes’ 2026 earnings per share have increased from $1.54 to $1.91. ALKS shares have lost 8.3% over the past year.
Alkermes’ earnings beat estimates in three of the trailing four quarters and missed in the remaining one, with the average earnings surprise being 4.58%.
Over the past 60 days, estimates for Castle Biosciences’ 2026 loss per share have narrowed from $1.06 to 96 cents. CSTL shares have risen 25.4% over the past year.
Castle Biosciences’ earnings beat estimates in three of the trailing four quarters and missed in the remaining one, with the average surprise being 66.11%.
Disclaimer: The content of this article solely reflects the author's opinion and does not represent the platform in any capacity. This article is not intended to serve as a reference for making investment decisions.
You may also like
Shiba Inu Will Recover to Prices Never Seen Before—Top Analyst

Cardano Price Analysis for Feb 18: Here Are Potential Case Scenarios for ADA Price

The XRP Flywheel Effect: Why Price Discovery May Become Inevitable as Corridors Flip

XRP Inflection Point Will Happen When Most Investors Are Looking in the Wrong Direction: Pundit